Your Immune System – Protection & Defence
It’s actually rather unfair: Some people never seem to get ill, while others suffer from a sore throat, urinary tract infections and digestive issues several times a year. These complaints are caused by germs which in many cases are transmitted directly from person to person or are ingested via the food we eat. However, even if these intruders attacks us, that doesn’t necessarily mean we will get ill. This is decided by our immune system – and the gut controls 80% of the immune system!
The immune system’s strategies
Nature has developed an ingenious defence system against diseases. In the course of evolution, it had to constantly “update” itself and develop new defence strategies in order to protect us from pathogenic germs. The immune system is sort of like a family-run business in which everyone must take on specific tasks. If there isn’t lots to do, then the immune cells tend to become a little lazy and relaxed. However, if the microbial enemy launches an attack, then everyone in the business knows what to do in order to protect the system: Everyone must pull together and act as one. The bacteria residing in the uppermost layer, hence the skin or mucous membranes, act as the first barrier against potential intruders. If there are enough well-meaning family members, or commensals (harmless bacteria), it is easy to do away with the intruder. However, if a troublemaker manages to overcome the first hurdle, for example by employing camouflage or deception, then it will make its way into the next level. Here the intruder is yet again met with a rebuff – or not. What this means for our immune system is that if pathogens reach the mucosa of the digestive tract (e.g. the mouth, gut, or stomach) then enzymes for the protection of the body, tiny hair cells called cilia, and epithelial cells make sure that the intruder is transported out of the body. The systemic interaction of innumerable lone fighters finally leads to a successful immune defence in our bodies – just like a well-coordinated team. In the human immune system cells known as leukocytes, also known as white blood cells, play an important role in the primary defence against pathogenic germs.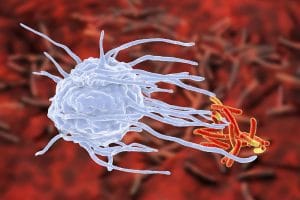
Leukocytes have lots of “relatives”. For example, neutrophilic granulocytes have the special capability to recognise a foreign attacker and save a profile of it. T- and B-lymphocytes have very special sensors, these are known as antigen receptors, which enable them to scout out and kill the enemy, e.g. foreign proteins. Luckily, special lymphocytes known as “memory cells” remember this for a lifetime. Dendritic cells can report the trespassing of a foreign protein. Thereupon, t-cells begin releasing cytokines (messenger substances) which initiate the destruction of the enemy. Monocytes also belong to the family of leukocytes. In the case of imminent danger, they turn into macrophages (devouring cells) and dismantle the enemy into its protein components. In turn, B- and T-cells can recognise these. If the body encounters the same pathogen again after days, weeks, or years, B-lymphocytes can immediately begin with the production of antibodies and the enemy (the antigen) is destroyed quickly and efficiently.
These “fights” go on within the lymph nodes of the body’s lymphatic system where the B- and T-cells can be found. These nodes are located for example on the neck, under the armpits, in the groin, or in the abdominal region. These B- and T-cells are assisted by dendritic cells which help to “transfer” the enemy so it can be destroyed more effectively. Additionally, B-cells produce immunoglobulins (antibodies) which specifically target the enemy and help to destroy it. Typically, immunoglobulin M (IgM) is produced in the early phase of an infection. Immunoglobulin G (IgG) can then be found in the blood during later phases of an infection. This means one can check for IgG in someone’s blood to determine whether someone has already come into contact with a specific pathogen.
Innate or acquired?
Parts of the body’s immune function are already present at birth and last a lifetime, such as the mechanism between the granulocytes, macrophages, and dendritic cells. However, the acquired immune system and its development is unique in every person. Depending how many germs an immune system encounters over the years and how many of these are recognised and “remembered” as being hazardous, decides how strong or weak it is. “In the course of a lifetime the body has the possibility to recognise, to remember, and to react to around a billion pathogenic germs”, says Dr. Adrian-Mathias Moser, physician and PhD student at the Medical University of Graz. The largest portion of our immune system is located in the gut. This makes sense, as the lion’s share of antigens gains access to our bodies via the gut – hence, they come from our food.
With its 300-400m² surface area the gut is our largest defence system as roughly 80% of all immune cells lie beneath the gut mucosa. “Three factors are important for the gut’s barrier function: a healthy gut mucosa, functional immune cells, and an intact microbiome (a favourable bacterial colonisation of the gut). An impaired gut flora can also mean that the immune system is weakened. Thus, a stable immune system depends greatly on the state of the microbiome”, explains Moser. “The sturdier the gut mucosa, the less likely it is that antigens (this term includes “bad” germs, pollutants, and unhealthy food components) gain access to the body’s circulation.” If they do manage to get that far, this can lead to immoderate inflammatory reactions throughout the body and so lay the foundation for autoimmune diseases such as multiple sclerosis, type 1 diabetes, and rheumatoid arthritis.
Environment and diet influence the immune system

Viruses and red blood cells
Factors which are harmful for our immune system develop due to e.g. a diet which is high in fat and sugar, diets lacking fibre, or even stress. If additionally, the gut comes under attack from harmful “antigens” then at some point the gut mucosa can no longer stave them all off – the entire immune system is weakened. The gut’s immune system consists of lymphatic tissue: on the one hand, mucosa associated lymphoid tissue (MALT), hence the mucous tissue which lines the insides of organ cavities and interconnects human organs. And on the other hand, it consists of the gut’s mucous tissue (GALT= Gut associated lymphoid tissue). Furthermore, it contains innumerable immune cells. For example, the gut has the highest proportion of B- and T-lymphocytes which are also present in other mucous tissues as well as secretions (tear fluid, vaginal secretions, or saliva) around the body and at the same time are interconnected through lymph nodes (e.g. on the neck, around the groin, stomach or under the armpits).
How a gut with a strong immune system fights
The principle behind immunity also applies to the gut: When it comes to the tissue of the gut mucosa, the defence begins in the epithelium of the small intestine which absorbs any pathogens from our food. Thereupon, resident macrophages dismantle them and present the fragments to T- and B- lymphocytes. T-helper cells produce proinflammatory cytokines (interleukins) which must remain in balance, while B-lymphocytes ravage the dismantled enemy: due to the production of immunoglobulin A the enemy is destroyed and so can no longer penetrate the gut mucosa. “A stable immune system strongly depends on the bacterial colonisation of the gut”, says Moser. Which bacteria end up colonising the gut depends on several factors: Primarily the birth process (hence the initial colonisation with bacteria), antibiotic intake, and scientists are discovering what an important role our nutrition plays for the development of the gut flora. For example, it has been shown that people who eat lots of animal products have a less diverse microbiome than people who primarily eat vegetables. It’s interesting that as soon as one changes one’s diet, other bacteria begin colonising the gut. And as long as the environment in the gut is good, then the new bacteria that come along are “good” bacteria. However, if you start consuming too much sugar or alcohol then it’s the “bad” germs that will multiply.
What does “healthy gut” even mean?
At the moment clinicians are working on figuring out this very question. They are trying to find a kind of “reference microbiota” with the help of highly complex analyses and this may open many exciting possibilities for the future of medicine. While the human genome was already determined in 2001, the investigation of the microbial genome began many years later. Just a comparison: the human genome contains roughly 22,000 genes, whereby, the bacteria living in the gut have an unimaginable diversity with about 3 million genes which communicate with human genes.
The immune system adapts to living standards
Many studies and investigations in the last years have shown that there is a constant lively exchange between the immune system and the gut flora (microbiome). “Obviously, evolution must have intended it to be this way so that over the course of time our immune defence could adapt to new environmental challenges”, says Moser. Judging by the newest findings, the composition and function of the microbiome play a role in the development of diseases such as multiple sclerosis, asthma, allergies, and other autoimmune diseases. A stable microbiome isn’t purely coincidence, the first steps towards one already begin in the womb. Investigations have shown that differences concerning the bacterial colonisation of babies depend on the way they were born. Babies which are born naturally, by a vaginal birth are colonised mostly by lactobacilli which are passed on from the mother’s mucous membranes. Babies born by caesarean section are mainly colonised with clostridium and staphylococcus strains which predominantly colonise the human skin. Lactobacilli are considered to strengthen the immune system. This explains why C-section babies are prone to allergies – because they are colonised by the wrong bacteria. Thus, a resilient microbiome also depends on the type of birth. This was also shown in a study from M.G. Dominguez from the University of Puerto Rico. “Presumably the first bacteria to inhabit the gut have a lifelong effect on the immune system”, confirms Moser. “Our gut flora is a self-contained ecosystem”, says the physician. Dendritic cells transmit signals to immune cells and for example the mesenteric lymph nodes (lymph nodes of the abdomen) – all lymph nodes in the human body are interconnected. Recently French scientists discovered that there even lymph nodes inside the brain. Antoine Loveau, researcher at the University of Virginia proved that a fine network of lymph vessels extends as far as the surface of the brain. This shows that the brain and immune system interact – this realisation may provide important information concerning the development of Alzheimer’s disease or multiple sclerosis.
Is there such thing as a “standard microbiome”?
How can we define a “good” microbiome? Moser on this topic: “One of the most important components is a broad diversity. This means that a more diverse bacterial colonisation gives rise to a more stable microbiome when it comes to fending off pathogens. The more intact an ecological system is, the more resilient it is, or rather, the defence against diseases improves.” This is reflected in the microbiomes of indigenous people which show far more diversity than the microbiomes of Europeans or populations of industrialised countries. C-sections, antibiotic intake, and fast food are all negative influence factors which explain the varying composition of different microbiomes.
A strong gut barrier is protective
A study from the Drug Research Institute in Leuwen, Belgium showed that the gut’s immune system is also involved in the development of type 2 diabetes and obesity. They saw that the intestinal immune system plays a key role in the development of the metabolic imbalances caused by a high-fat diet. In this regard, a specific gut protein (MyD88) plays an integral role in the creation of fat deposits. The researchers showed that the modification of the gut colonisation led to a deactivation of this protein, which in turn led to a strengthening of the intestinal barrier. This resulted in less inflammation, a slowing of weight gain, and protection against diabetes development. Thus, the gut immune system also plays an integral role in the regulation of fat retention.


Dr. Adrian-Mathias Moser
The gut immune system and autoimmune diseases
Moser is currently investigating the role which the immune system, or rather, the microbiome plays regarding multiple sclerosis. To do this, tests were done with mucosal cells which were cultivated in a lab. It is currently being evaluated which immune cells fail to defend the body and if this circumstance may finally lead to the development of multiple sclerosis. In 20-25% of cases, the disease develops due to a genetic disposition. First studies regarding diseases such as asthma, rheumatoid arthritis, diabetes, and other autoimmune diseases suspect that similar principles may be at play in the development of these diseases. There are similar speculations regarding Parkinson’s and Alzheimer’s disease. Besides genetic factors, “microbiological genetics” are now coming into play. Which raises the question: Which proportion of these components originate in the gut?
Nature is diverse
“Everything which leads to a loss of biodiversity, also effects humans”, says Moser. “In the future, we should view the gut flora more from an ecological standpoint: Food from natural sources, originating from good soil, vegetables from organic farming with a diverse flora are more beneficial than industrially produced foods which are oftentimes contaminated with pesticides or originate from animals which were fed with antibiotics.” As a physician who practices preventative medicine he emphasises that: “Tolerance is not only a field of sociology. In a figurative sense, it is also a wide field of ecology. That what grows on such fertile ground, is diversity. And it is diversity which keeps us healthy.”
Around 80% of all immune cells lie within the gut. Pathogenic “attackers” are repelled by the gut flora, the gut mucosa, and the immune cells beneath the gut mucosa. If the gut’s barrier function is impaired, then these pathogenic germs can penetrate the body via the gut and can so give rise to diseases and weaken general immunity. A dysfunctional filter function can cause diseases such as allergies, intolerances, inflammation of the gut mucosa, and digestive problems.
Positive influences which the microbiome has on the immune system
Plant-based food is rich in short-chain fatty acids (SCFA). SCFAs stimulate anti-inflammatory cells in the gut mucosa. Animal foods only contains very small amounts of SCFAs; this is closely linked to immunological diseases (e.g. chronic inflammatory bowel diseases). Calorie reduction strengthens the immunes system – at least in mice. Studies have shown that mice consuming a low-calorie diet produce fewer inflammatory markers, have a more distinct bacterial diversity, and a stronger intestinal wall which prevents germs from entering the blood stream.